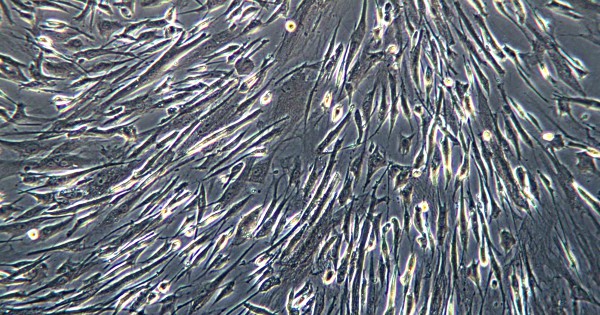
Mesenchymal stem cells (MSCs) are multipotent stem cells that are particularly of interest due to their unique ability to differentiate into a variety of cell types. They are obtained from within various body tissues including umbilical cord tissue and blood. MSCs have the capability to treat a variety of diseases, the full extent of which is still being explored. These stem cells can be used for autologous and even allogeneic transplants, without the risk of being rejected by the system or GVHD.
Umbilical cord blood and tissue derived MSCs carry may unique advantages over other sources such as easy procurement, no risk for the donors, no ethical issue as opposed to fetal or embryonic stem cells, no risk of rejection or GVHD, and no risk of unlimited growth or tumor regeneration. Moreover, abundant quantities of MSCs are present in cord blood and tissue, making this cell source unique.
Research into the field of mesenchymal stem cells is particularly beneficial to pharmaceutical companies. MSCs can be applied to more efficient targeting and delivery of drugs to damaged sites. They hold many advantages over other types of stem cells due to their ability to target inflammatory sites caused by tissue injury, regulate immune functions, encourage the repair of injured cells and prevent inflammation.
Currently at Beike Biotechnology, we are exploring a diverse range of applications of mesenchymal stem cells. Most recently, Beike Biotechnology along with leading Chinese hospitals and universities published research that showed that MSCs showed the ability to treat heart attack. Another milestone was the use of an integrated system of mesenchymal stem cells paired with an epidural stimulator to treat spinal cord injury.
With 128 trials globally that involve MSCs, over one third of which are centred around the application of these for disease indicators, the future for mesenchymal stem cells looks extremely promising.
| Beike’s UCBSC | Stromal Vascular Function | Other’s UCBSC | Embryonic/Fetal Stem Cells |
|---|---|---|---|
| High quantity (300-400 million cells) | Very low quantity | Low/undetermined quantity | Low/undetermined quantity |
| High viability | High viability | Low/undetermined viability | Low/undetermined viability |
| Known way of migration towards the injury site | Known way of migration towards the injury site | Known way of migration towards the injury site | Possibility of inappropriate stem cell migration and complications |
| Known differentiation potential | Known differentiation potential | Known differentiation potential | Poorly understood/unlimited differentiation potential and risk of complications |
| Differentiate into specific/targeted cell types | Differentiate into specific/targeted cell types | Differentiate into specific/targeted cell types | Differentiate into almost all/ untargeted cell types and risk of complications |